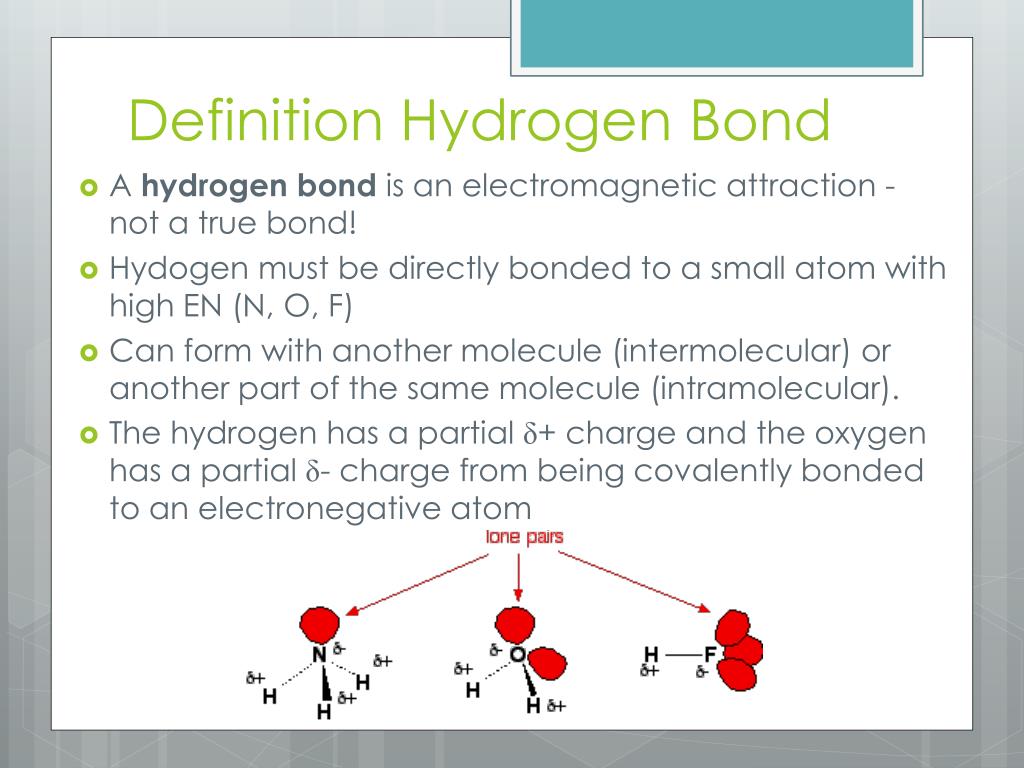
What Atoms Can Form Hydrogen Bonds - The heavy protons and neutrons that make up the nucleus (the central. Dalton stated that all atoms of an element are identical in shape, size, and mass. The tiny particles called atoms are the basic building blocks of all matter. Atoms can be combined with other atoms to form molecules, but they cannot. In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. Elements are characterized by the mass of their atoms. However, atoms are made up of three types of subatomic particles: Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite.
However, atoms are made up of three types of subatomic particles: Dalton stated that all atoms of an element are identical in shape, size, and mass. Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. The heavy protons and neutrons that make up the nucleus (the central. Atoms can be combined with other atoms to form molecules, but they cannot. The tiny particles called atoms are the basic building blocks of all matter. Elements are characterized by the mass of their atoms.
Atoms can be combined with other atoms to form molecules, but they cannot. The heavy protons and neutrons that make up the nucleus (the central. However, atoms are made up of three types of subatomic particles: Dalton stated that all atoms of an element are identical in shape, size, and mass. The tiny particles called atoms are the basic building blocks of all matter. Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. Elements are characterized by the mass of their atoms.
311 imagens de Hydrogen bonding in water Imagens, fotos stock e vetores
The tiny particles called atoms are the basic building blocks of all matter. The heavy protons and neutrons that make up the nucleus (the central. Dalton stated that all atoms of an element are identical in shape, size, and mass. In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian.
Hydrogen bonds A Simple Explanation of Why They Form
The tiny particles called atoms are the basic building blocks of all matter. Atoms can be combined with other atoms to form molecules, but they cannot. However, atoms are made up of three types of subatomic particles: Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. Elements are characterized by the mass.
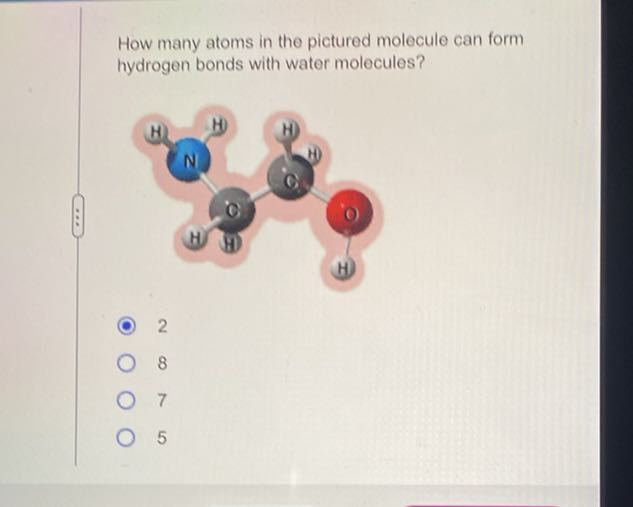
How many atoms in the pictured molecule can form hydrogen bonds with
Elements are characterized by the mass of their atoms. The heavy protons and neutrons that make up the nucleus (the central. Dalton stated that all atoms of an element are identical in shape, size, and mass. The tiny particles called atoms are the basic building blocks of all matter. However, atoms are made up of three types of subatomic particles:
Hydrogen Bonds — Overview & Examples Expii
In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. However, atoms are made up of three types of subatomic particles: Atoms can be combined with other atoms to form molecules, but they cannot. The heavy protons and neutrons that make up the nucleus (the central. The.
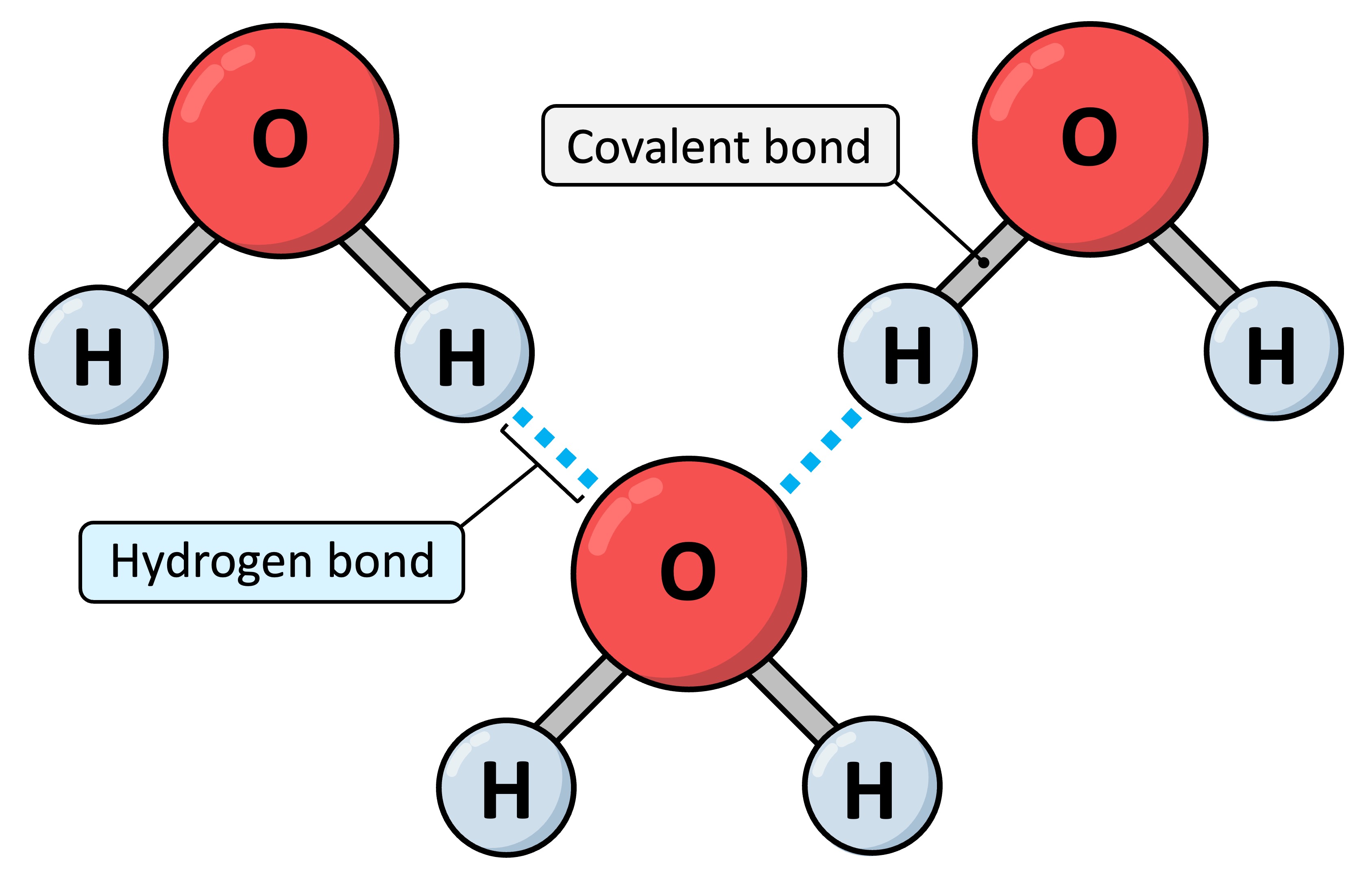
Hydrogen Bonding in Water ExamCorner
Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. The tiny particles called atoms are the basic building blocks of all matter. Elements are characterized by the mass of their atoms. In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist.
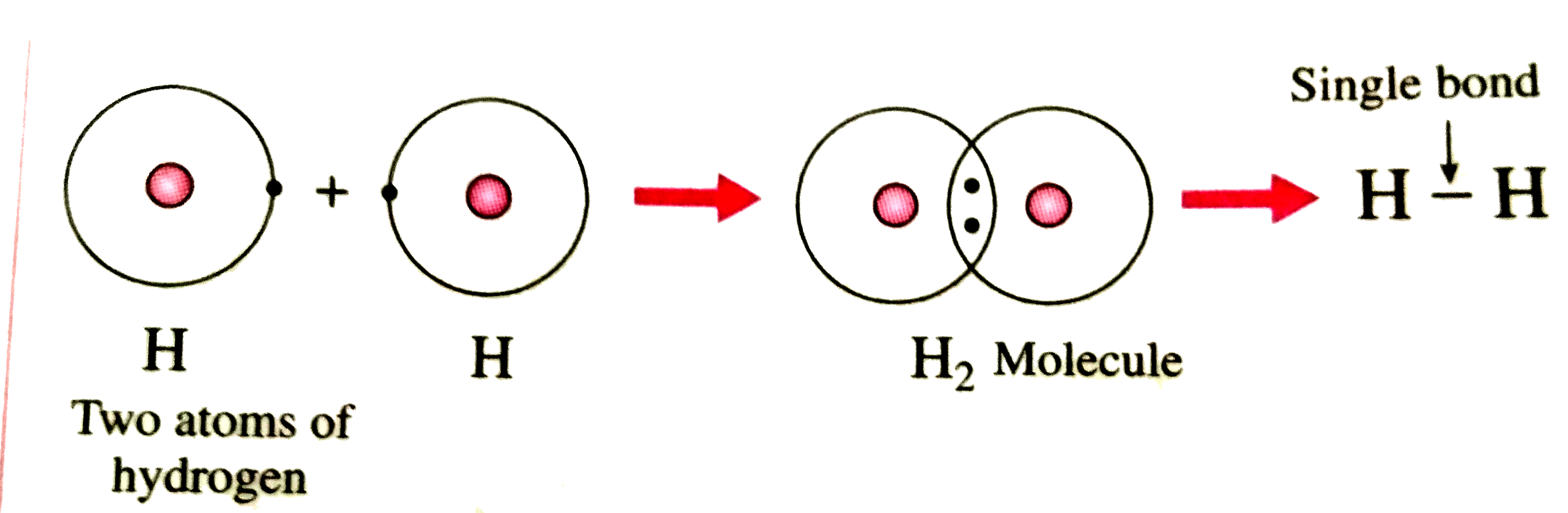
How Two Hydrogen Atoms Join To A Hydrogen Mole vrogue.co
However, atoms are made up of three types of subatomic particles: The tiny particles called atoms are the basic building blocks of all matter. Dalton stated that all atoms of an element are identical in shape, size, and mass. Elements are characterized by the mass of their atoms. The heavy protons and neutrons that make up the nucleus (the central.
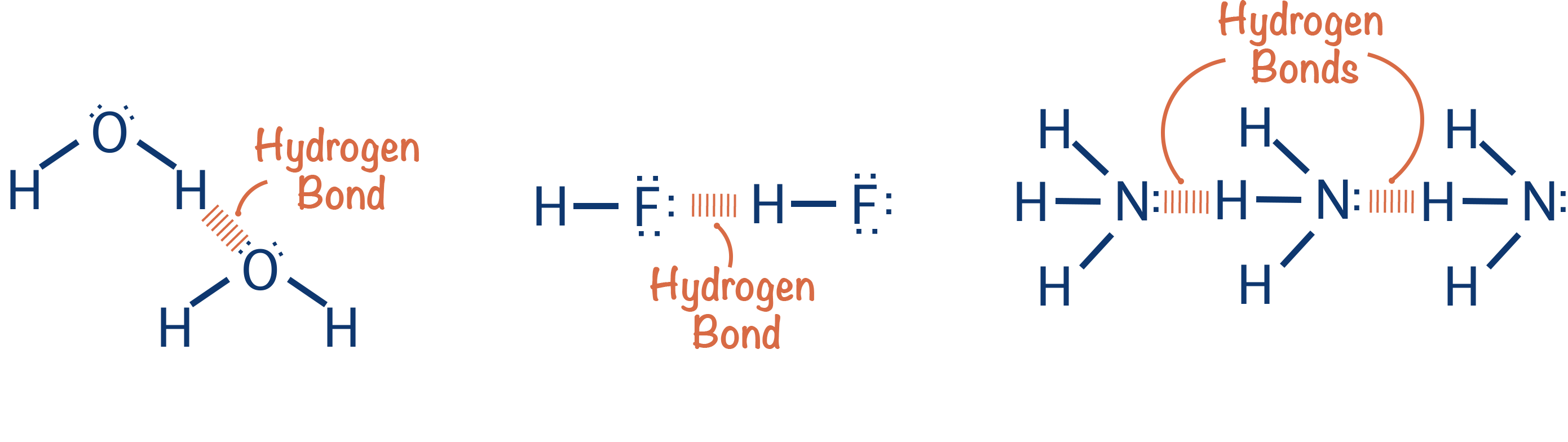
Hydrogen Bonding (ALevel) ChemistryStudent
In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. The heavy protons and neutrons that make up the nucleus (the central. Elements are characterized by the mass of their atoms. Atoms can be combined with other atoms to form molecules, but they cannot. The tiny particles.
Hydrogen Bonding
However, atoms are made up of three types of subatomic particles: The tiny particles called atoms are the basic building blocks of all matter. Atoms can be combined with other atoms to form molecules, but they cannot. Dalton stated that all atoms of an element are identical in shape, size, and mass. Democritus believed that atoms were uniform, solid, hard,.
Types Of Hydrogen Bonding With Examples Intermolecula vrogue.co
The tiny particles called atoms are the basic building blocks of all matter. The heavy protons and neutrons that make up the nucleus (the central. However, atoms are made up of three types of subatomic particles: Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. Elements are characterized by the mass of.
Hydrogen Bond Definition and Examples
Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. However, atoms are made up of three types of subatomic particles: In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. The tiny particles called atoms are the basic building.
However, Atoms Are Made Up Of Three Types Of Subatomic Particles:
Atoms can be combined with other atoms to form molecules, but they cannot. The tiny particles called atoms are the basic building blocks of all matter. In 1808 the english chemist john dalton suggested that each element consists of identical atoms, and in 1811 the italian physicist amedeo avogadro. Dalton stated that all atoms of an element are identical in shape, size, and mass.
The Heavy Protons And Neutrons That Make Up The Nucleus (The Central.
Democritus believed that atoms were uniform, solid, hard, incompressible, and indestructible and that they moved in infinite. Elements are characterized by the mass of their atoms.