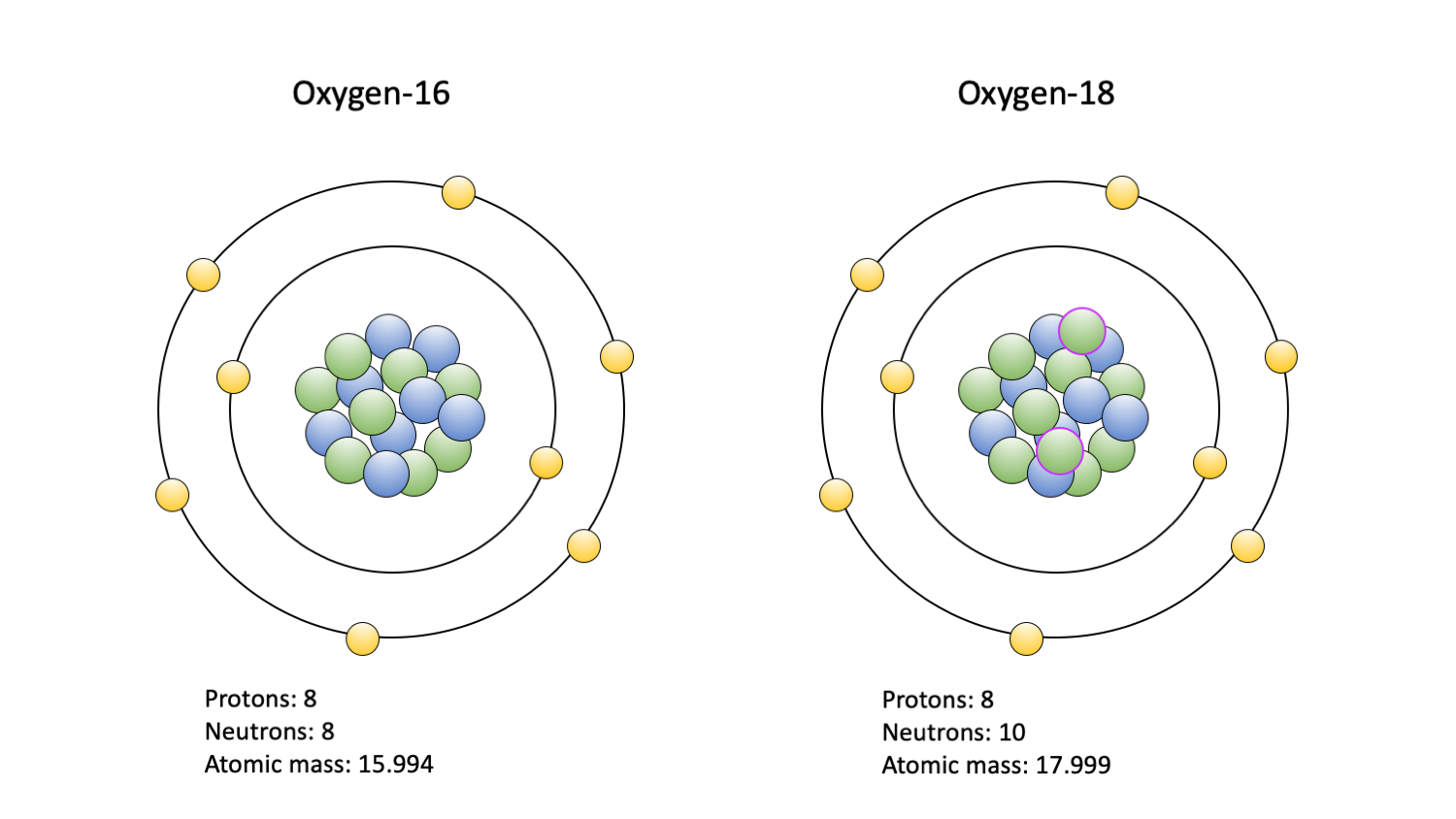
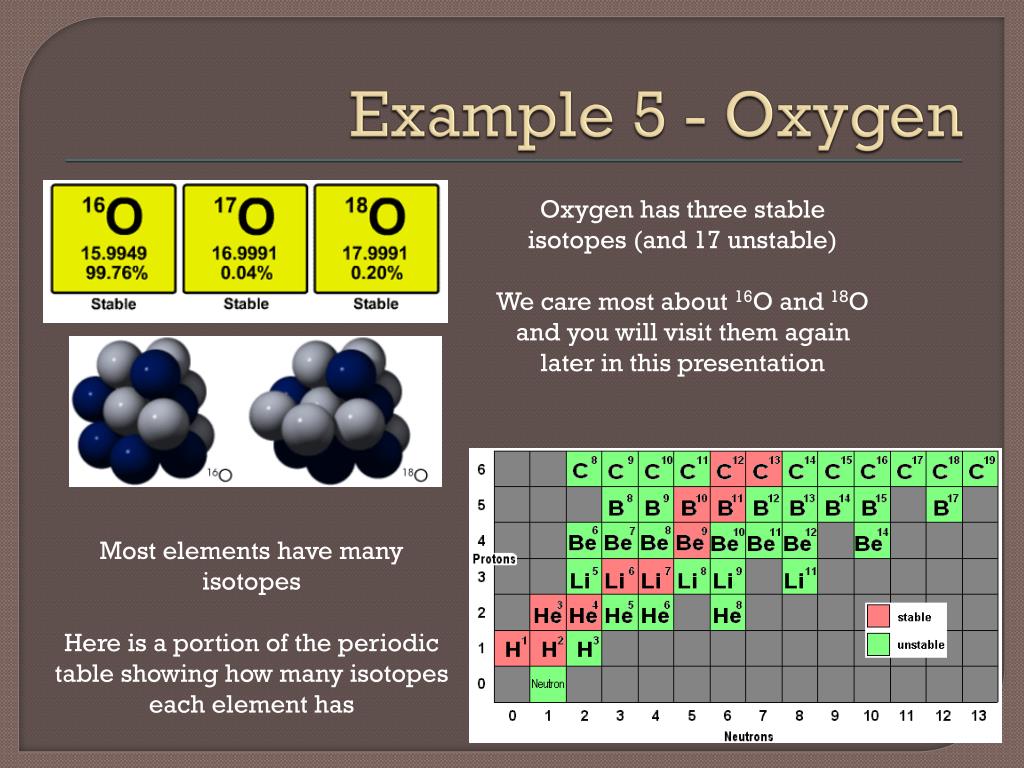
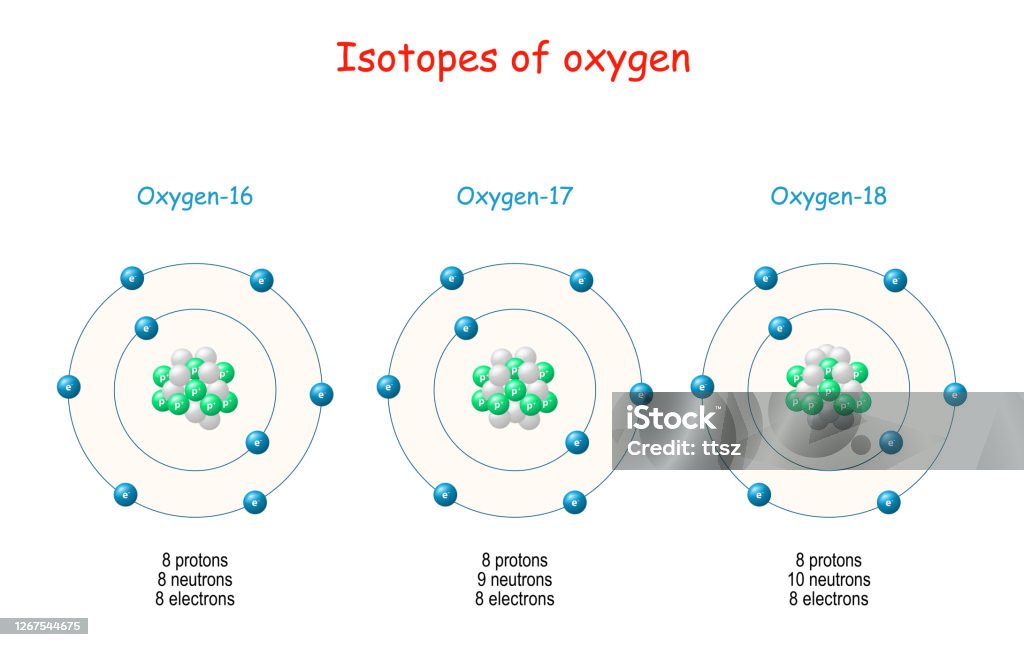
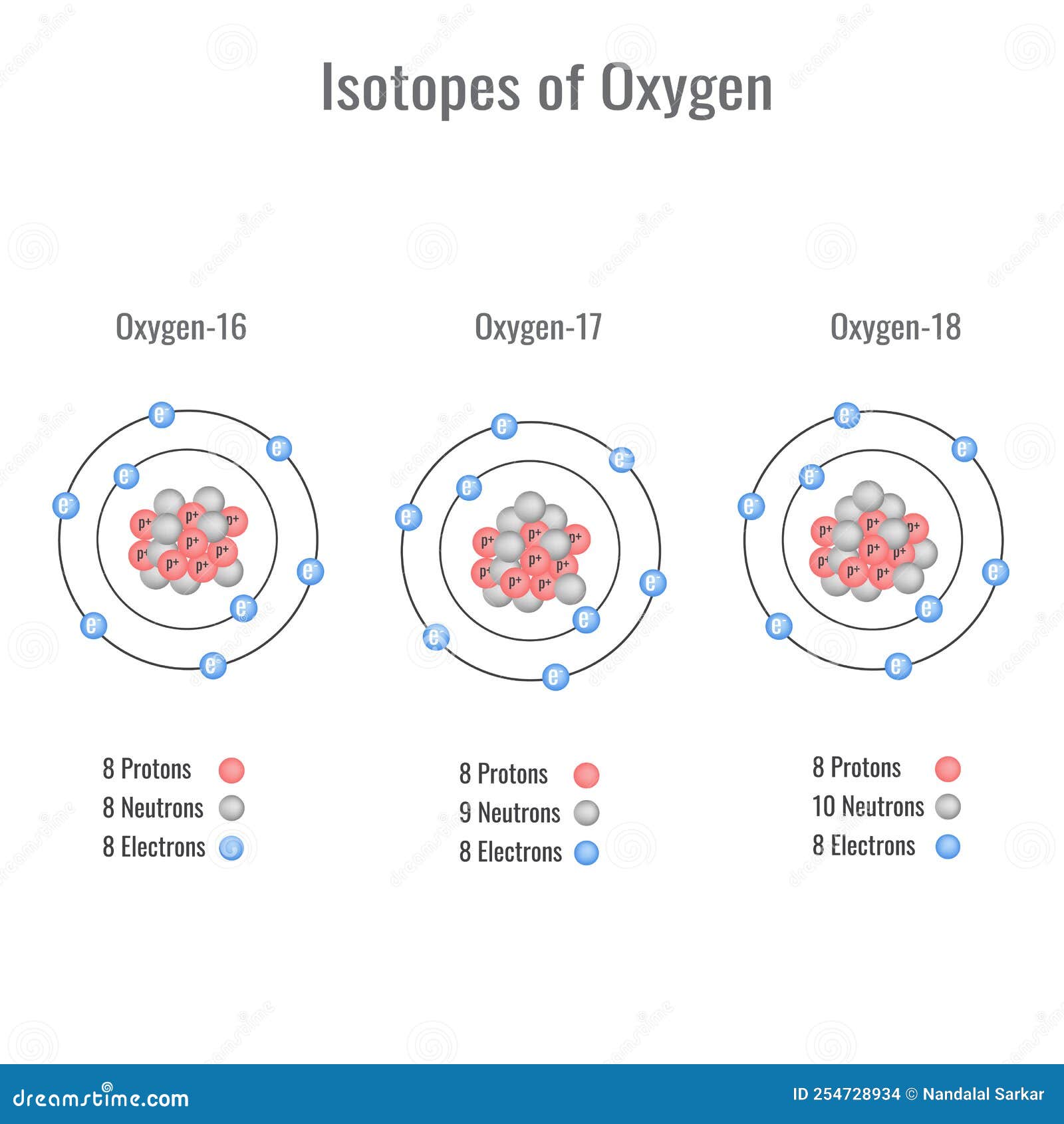
Stable Forms Of Oxygen - Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. What is isotope oxygen is? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of oxygen? No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to.
Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oh, dude, you're talking about the stable forms of oxygen? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. What is isotope oxygen is? No, oxygen typically forms 2 bonds.
What is isotope oxygen is? Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. No, oxygen typically forms 2 bonds. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of oxygen? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to.
Stable Atom Over 400 RoyaltyFree Licensable Stock Vectors & Vector
Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oh, dude, you're talking about the stable forms of oxygen? What is isotope oxygen is? No, oxygen typically forms 2 bonds.
Biogeochemical analysis and Paleoecology Digital Atlas of Ancient Life
Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. No, oxygen typically forms 2 bonds. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons.
Ionic Bonding. ppt download
No, oxygen typically forms 2 bonds. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oh, dude, you're talking about the stable forms of oxygen? What is isotope oxygen is?
Thermochemistry Thermodynamics Gases Paul Franklyn C305 Consultation
No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oh, dude, you're talking about the stable forms of oxygen? Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oxygen atoms need to.
PPT Isotopes, Ice Cores and Climate Change PowerPoint Presentation
Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of oxygen? No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oxygen atoms need to.
Isotopes Of Oxygen Stock Illustration Download Image Now Isotope
Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oxygen atoms need to share or gain two electrons.
Isotope Symbol
No, oxygen typically forms 2 bonds. Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. What is isotope oxygen is? Oh, dude, you're talking about the stable forms of oxygen?
Element ' X ' forms five stable oxides with oxygen of formula X2 O,XO2 ,X..
What is isotope oxygen is? No, oxygen typically forms 2 bonds. Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of oxygen?
Isotopes de l'oxygène illustration de vecteur. Illustration du chemical
Oh, dude, you're talking about the stable forms of oxygen? Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? No, oxygen typically forms 2 bonds.
PPT Trace Elements Definitions PowerPoint Presentation, free
Oxygen atoms need to share or gain two electrons in order to achieve a stable electron configuration. Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. What is isotope oxygen is? Oh, dude, you're talking about the stable forms of oxygen? Oxygen is in group 16 of the periodic table,.
No, Oxygen Typically Forms 2 Bonds.
Isotope oxygen is a variant of the element oxygen that has a different number of neutrons in its nucleus. Oh, dude, you're talking about the stable forms of oxygen? Oxygen is in group 16 of the periodic table, so it has 6 valence electrons and can gain 2 electrons to. What is isotope oxygen is?