Nf3 Form - Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. Nf3 is a polar molecule. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. No, nf3 is not an ionic compound. 1.nf3 develops a piece instead of moving a pawn to begin the game. It is a covalent compound where nitrogen (n) and fluorine (f) atoms share electrons to form bonds. It's actually considered the main line. The knight develops to a good square that controls the center while keeping. Wikipedia gives a better rundown,.
Nf3 is a polar molecule. It's actually considered the main line. It is a covalent compound where nitrogen (n) and fluorine (f) atoms share electrons to form bonds. The knight develops to a good square that controls the center while keeping. Wikipedia gives a better rundown,. No, nf3 is not an ionic compound. 1.nf3 develops a piece instead of moving a pawn to begin the game. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,.
It is a covalent compound where nitrogen (n) and fluorine (f) atoms share electrons to form bonds. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. Wikipedia gives a better rundown,. Nf3 is a polar molecule. 1.nf3 develops a piece instead of moving a pawn to begin the game. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. The knight develops to a good square that controls the center while keeping. No, nf3 is not an ionic compound. It's actually considered the main line.
NF3
The knight develops to a good square that controls the center while keeping. 1.nf3 develops a piece instead of moving a pawn to begin the game. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. Nf3 is a polar molecule. This is because the electronegativity difference.
NYS Form NF3 ≡ Fill Out Printable PDF Forms Online
It's actually considered the main line. Wikipedia gives a better rundown,. No, nf3 is not an ionic compound. The knight develops to a good square that controls the center while keeping. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven.
Nf3 Form Fillable ≡ Fill Out Printable PDF Forms Online
This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. The knight develops to a good square that controls the center while keeping. No, nf3 is not an ionic compound. 1.nf3 develops.
Nitrogen Trifluorid Nf3 Molecule Royalty Free Vector Image, 59 OFF
This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. No, nf3 is not an ionic compound. It's actually considered the main line. The knight develops to a good square that controls the center while keeping. 1.nf3 develops a piece instead of moving a pawn to begin the game.
NF3
Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. No, nf3 is not an ionic compound. 1.nf3 develops a piece instead of moving a pawn to begin the game. Wikipedia gives a better rundown,. It is a covalent compound where nitrogen (n) and fluorine (f) atoms.
Nf 3 Form Fill Out and Sign Printable PDF Template airSlate SignNow
This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. 1.nf3 develops a piece instead of moving a pawn to begin the game. Nf3 is a polar molecule. Wikipedia gives a better rundown,. It's actually considered the main line.
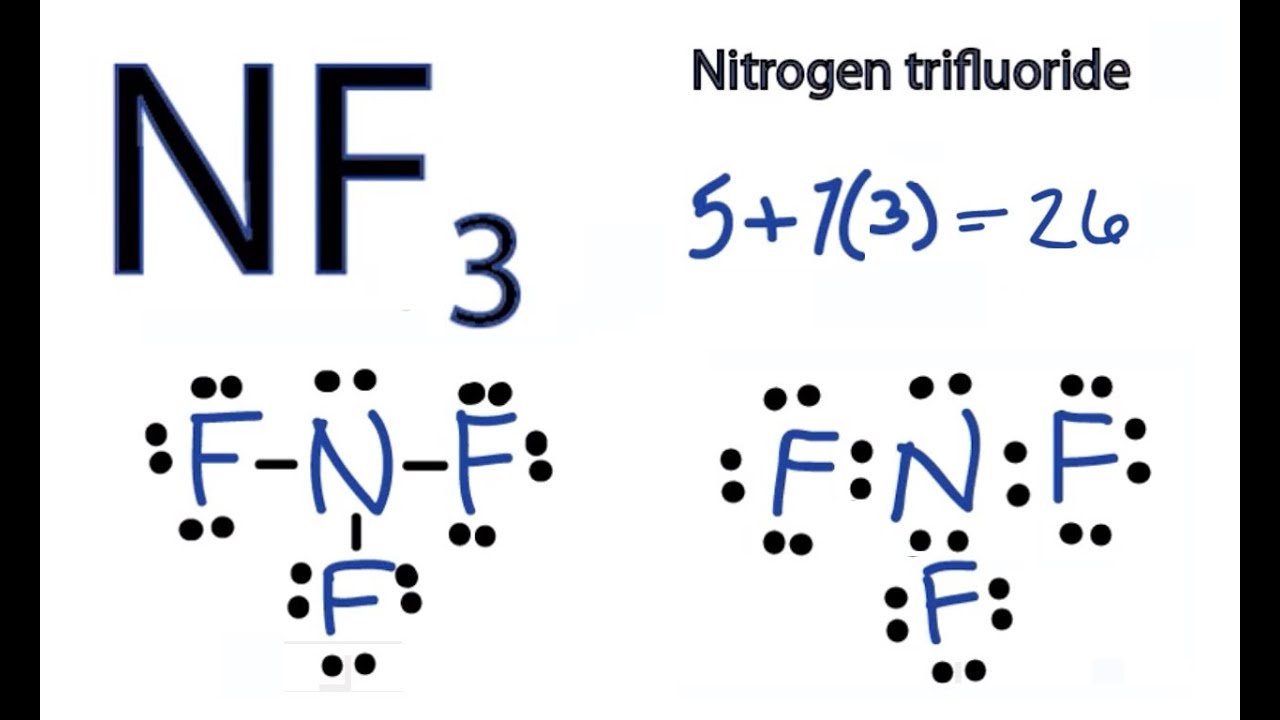
Draw The Lewis Structure Of The Molecule Nf3
It is a covalent compound where nitrogen (n) and fluorine (f) atoms share electrons to form bonds. It's actually considered the main line. Wikipedia gives a better rundown,. No, nf3 is not an ionic compound. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,.
No Fault Nf3 20092025 Form Fill Out and Sign Printable PDF Template
It is a covalent compound where nitrogen (n) and fluorine (f) atoms share electrons to form bonds. Nf3 is a polar molecule. It's actually considered the main line. No, nf3 is not an ionic compound. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven.
Unveiling the Lewis Dot Diagram for NF3 Understanding the Bonding
This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. No, nf3 is not an ionic compound. It's actually considered the main line. The knight develops to a good square that controls.
Fillable Online nf3 form download Fax Email Print pdfFiller
It's actually considered the main line. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. The knight develops to a good square that controls the center while keeping. 1.nf3 develops a.
It Is A Covalent Compound Where Nitrogen (N) And Fluorine (F) Atoms Share Electrons To Form Bonds.
Nf3 is a polar molecule. Wikipedia gives a better rundown,. Nf3, on the other hand, does not readily accept protons in water due to the presence of fluorine atoms that strongly attract electrons,. This is because the electronegativity difference between nitrogen and fluorine atoms leads to an uneven.
It's Actually Considered The Main Line.
1.nf3 develops a piece instead of moving a pawn to begin the game. The knight develops to a good square that controls the center while keeping. No, nf3 is not an ionic compound.