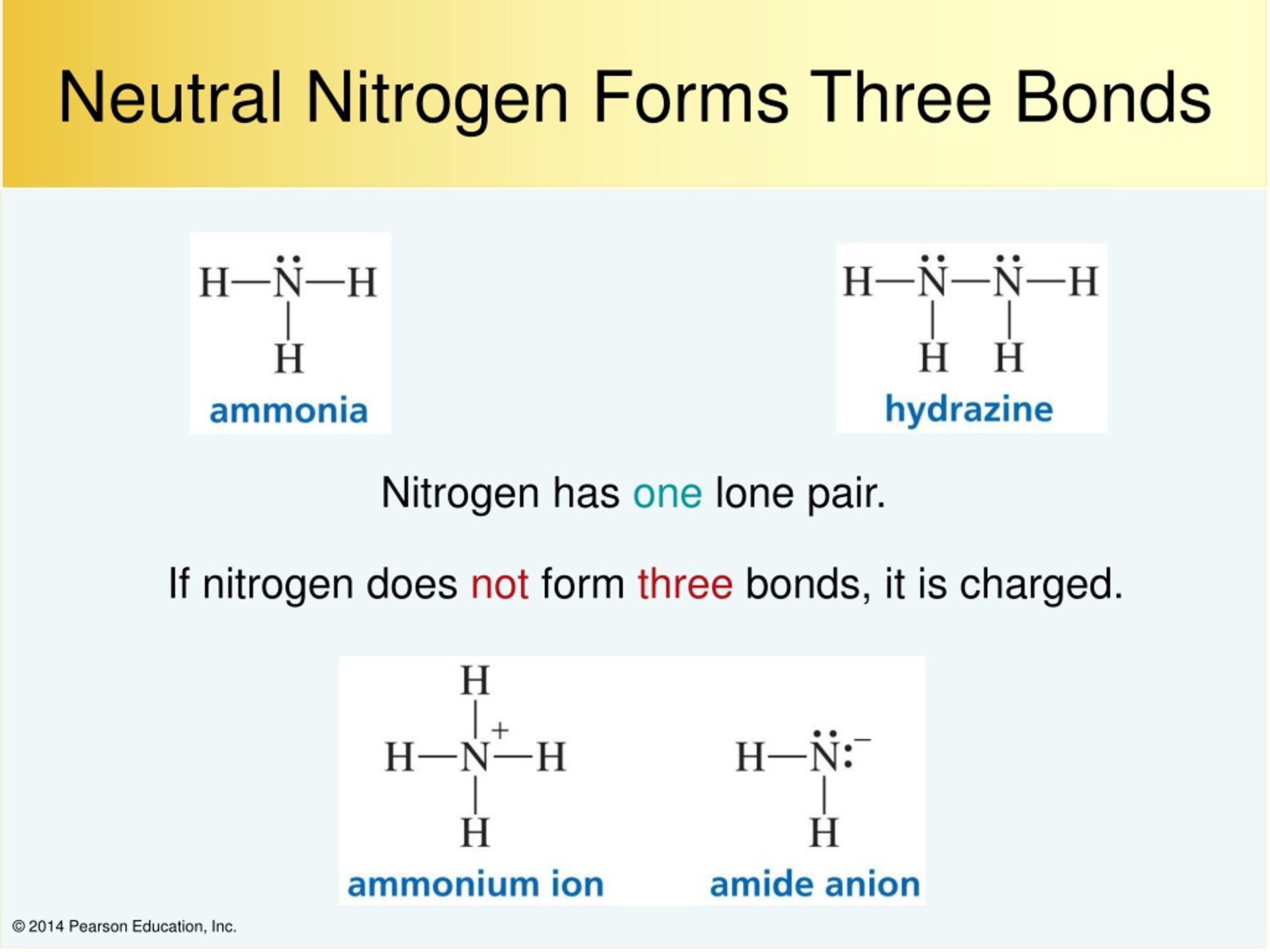
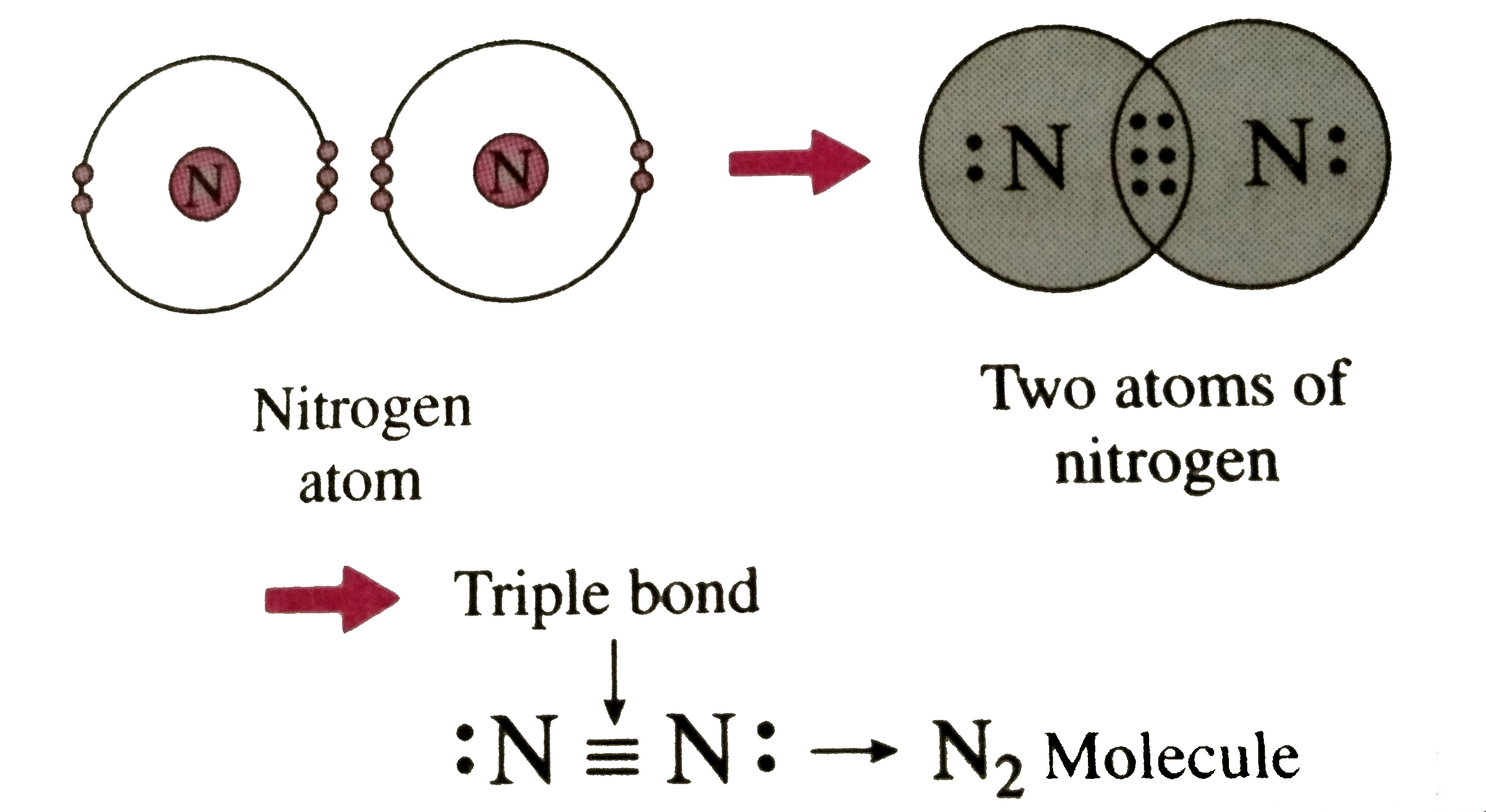How Many Bonds Does N Form - How many covalent bonds are in the element sb? However, with a positive net charge,. Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. K and cl would form an ionic bond as well. An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. Xenon (xe) typically forms covalent bonds. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. It is a noble gas and tends not to gain or lose electrons.
Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. Xenon (xe) typically forms covalent bonds. An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. K and cl would form an ionic bond as well. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. How many covalent bonds are in the element sb? However, with a positive net charge,. It is a noble gas and tends not to gain or lose electrons.
Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. How many covalent bonds are in the element sb? K and cl would form an ionic bond as well. Xenon (xe) typically forms covalent bonds. However, with a positive net charge,. It is a noble gas and tends not to gain or lose electrons.
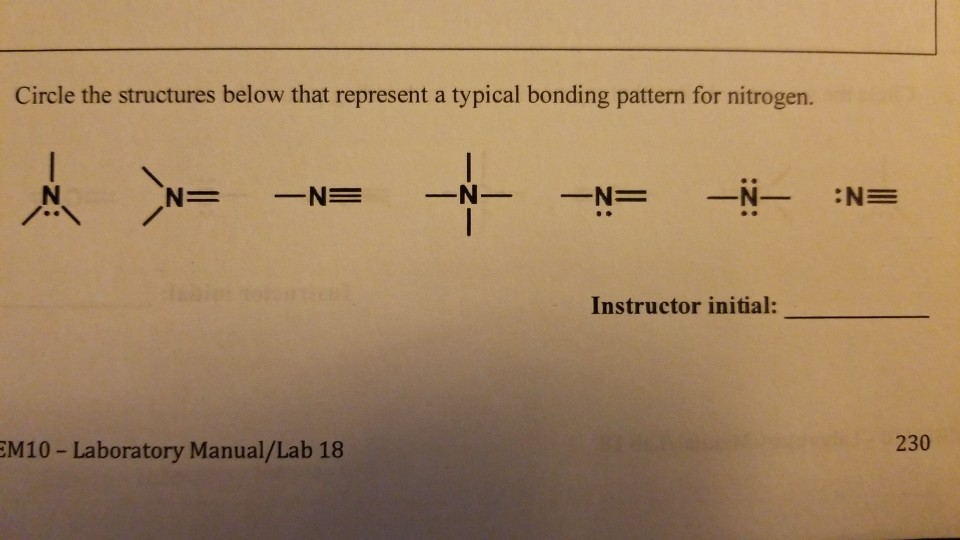
SOLVED How many bonds does nitrogen typically form? Does this make
Xenon (xe) typically forms covalent bonds. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. However, with a positive net charge,. Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen,.
Nitrogen Covalent Bond
How many covalent bonds are in the element sb? An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. Xenon (xe) typically forms covalent bonds. Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. In its pure form as an element, antimony (sb) is.
Bond formation in nitrogen molecule Stock Image C028/6484 Science
Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. Xenon (xe) typically forms covalent bonds. However, with a positive net charge,. An atom of nitrogen typically forms 3 covalent bonds to achieve.
Describe the formation of nitrogen molecule.
K and cl would form an ionic bond as well. However, with a positive net charge,. Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. An atom of nitrogen typically forms 3.
Solved How many bonds does nitrogen typically form? Does
It is a noble gas and tends not to gain or lose electrons. K and cl would form an ionic bond as well. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. Xenon (xe) typically forms covalent bonds. How many covalent bonds are in the element sb?
LabXchange
It is a noble gas and tends not to gain or lose electrons. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. Xenon (xe) typically forms covalent bonds. K and cl would form an ionic bond as well. However, with a positive net charge,.
PPT Remembering General Chemistry Electronic Structure and Bonding
Xenon (xe) typically forms covalent bonds. Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. However, with a positive net charge,. How many covalent bonds are in the element sb? It is a noble gas and tends not to gain or lose electrons.
Covalent Bond N2
It is a noble gas and tends not to gain or lose electrons. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. However, with a positive net charge,. K and cl would form an ionic bond as well. Xenon (xe) typically forms covalent bonds.
PPT Remembering General Chemistry Electronic Structure and Bonding
However, with a positive net charge,. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a. Hydrogen bonds form between a hydrogen atom bonded to an electronegative atom (such as oxygen, nitrogen, or fluorine) and another. How many covalent bonds are in the element sb? Xenon (xe) typically forms covalent bonds.
Nitrogen Covalent Bond
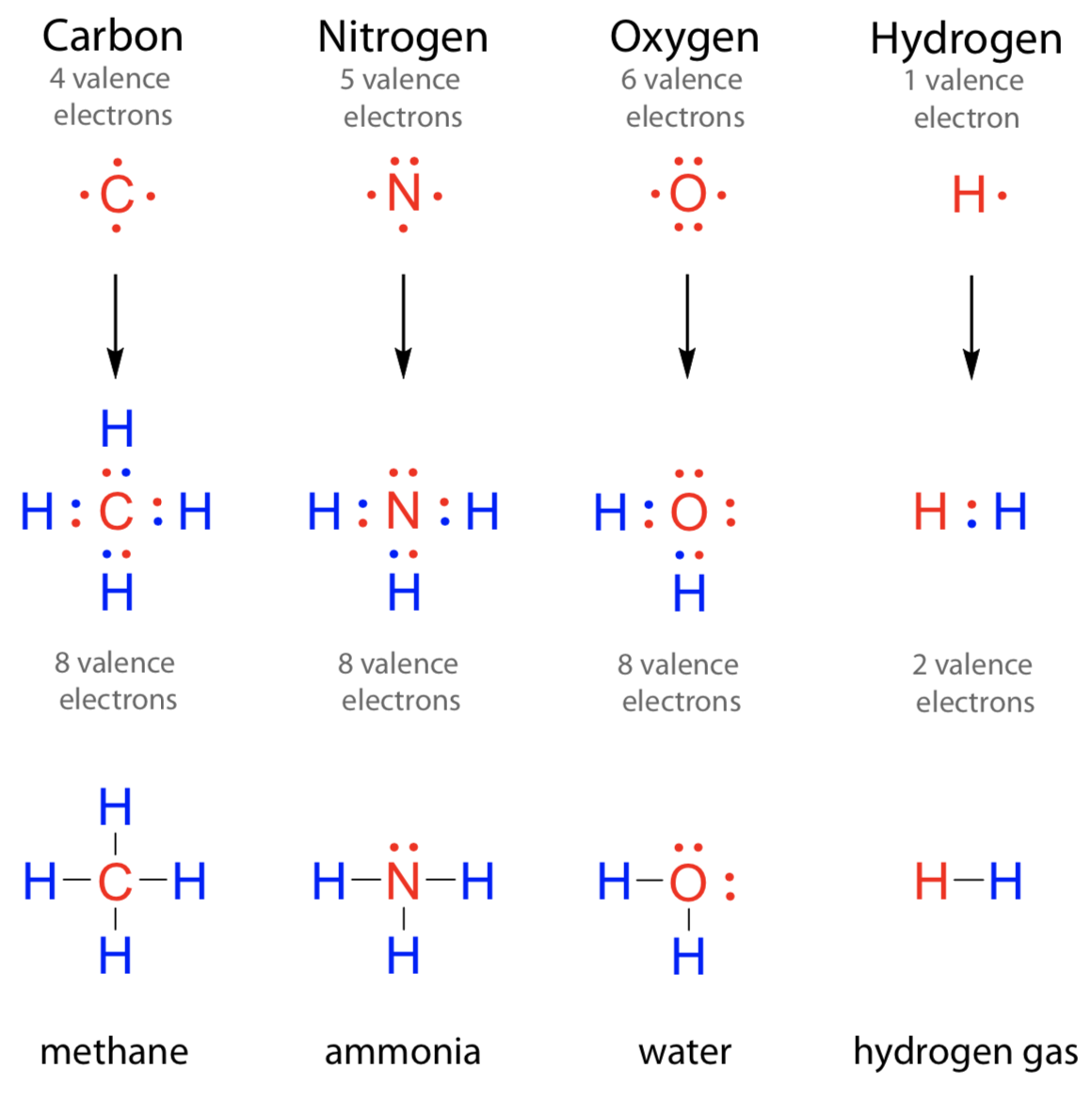
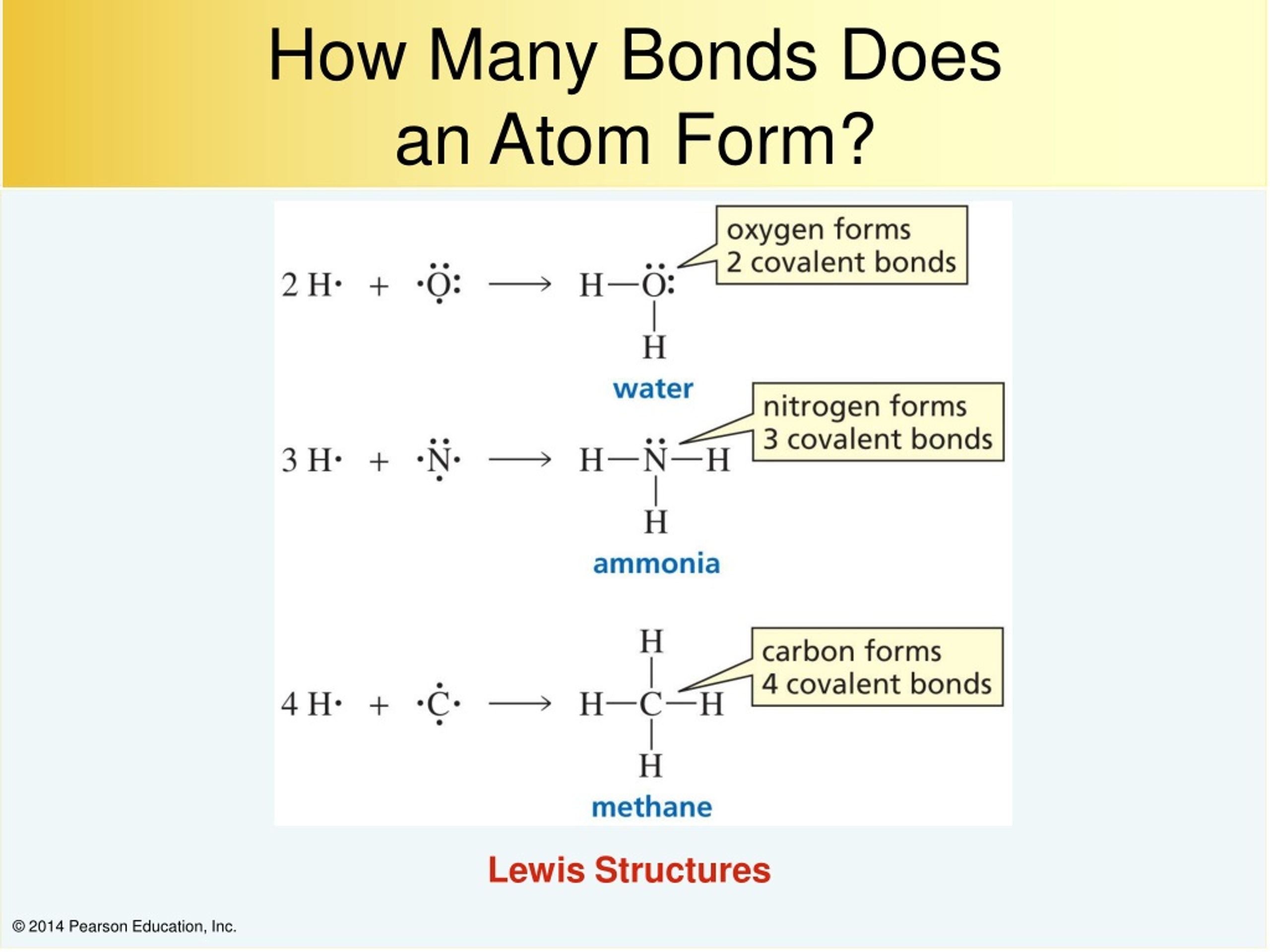
An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. It is a noble gas and tends not to gain or lose electrons. How many covalent bonds are in the element sb? Xenon (xe) typically forms covalent bonds. K and cl would form an ionic bond as well.
Hydrogen Bonds Form Between A Hydrogen Atom Bonded To An Electronegative Atom (Such As Oxygen, Nitrogen, Or Fluorine) And Another.
K and cl would form an ionic bond as well. How many covalent bonds are in the element sb? It is a noble gas and tends not to gain or lose electrons. In its pure form as an element, antimony (sb) is a metal, and it therefore forms a.
However, With A Positive Net Charge,.
An atom of nitrogen typically forms 3 covalent bonds to achieve a stable electron configuration. Xenon (xe) typically forms covalent bonds.