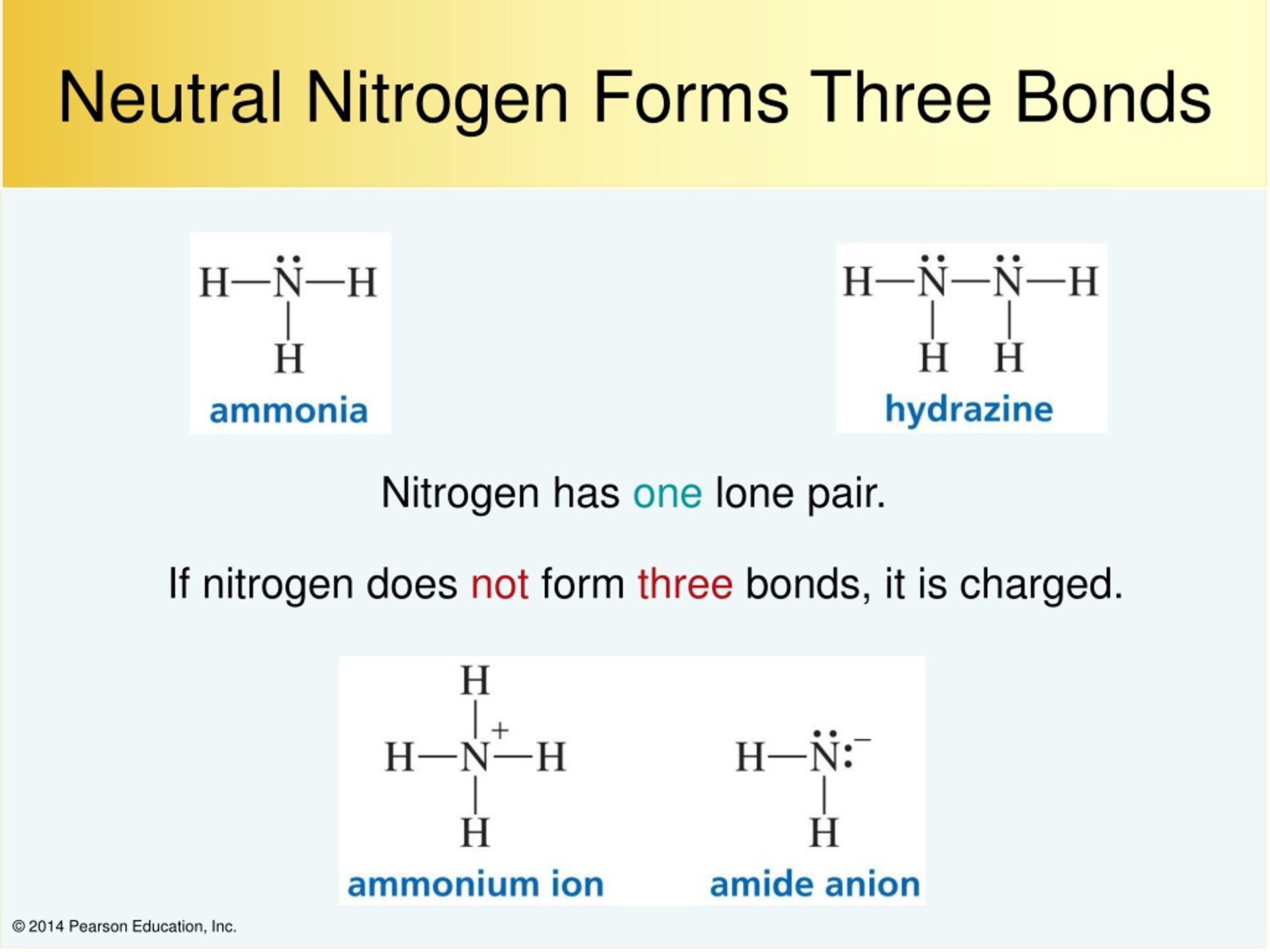
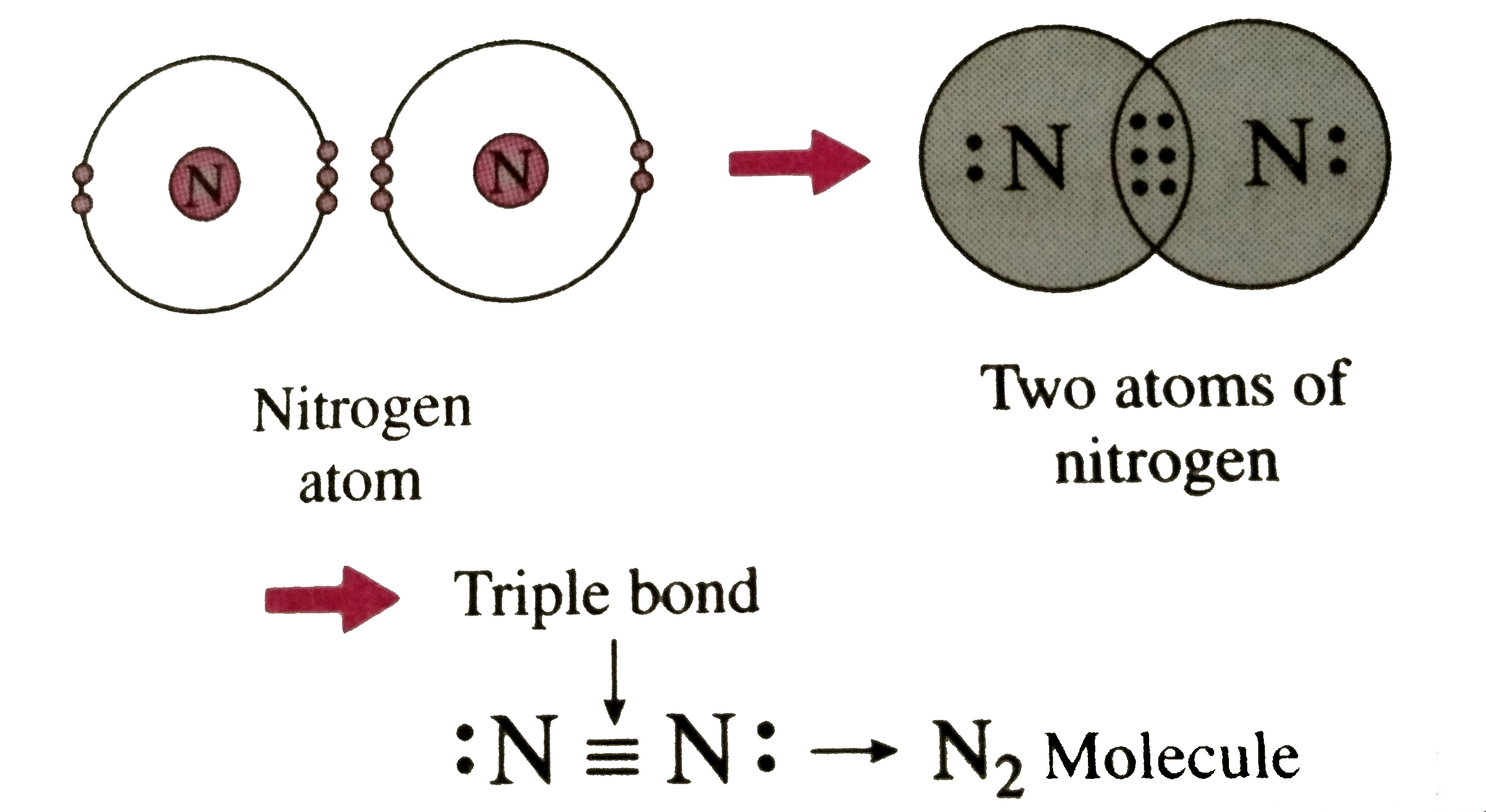How Many Bonds Can Nitrogen Form - How many covalent bonds are in ammonia? Nitrogen is in group 5 and therefoe has 5 outer electrons. Ammonia is a nitrogen atom bonded to three hydrogen atoms. There is a total of three covalent. Water molecules have two simple covalent bonds between one oxygen and two hydrogen. No, nitrogen and phosphorus would not typically form a covalent bond with each. Nitrogen can form 3 covalent bonds and 1 coordinate bond. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. How many covalent bonds does water have? Would nitrogen and phosphorus form a covalent bond?
There is a total of three covalent. Water molecules have two simple covalent bonds between one oxygen and two hydrogen. Ammonia is a nitrogen atom bonded to three hydrogen atoms. Nitrogen is in group 5 and therefoe has 5 outer electrons. Would nitrogen and phosphorus form a covalent bond? As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. How many covalent bonds are in ammonia? That means it needs 3 bonds. Nitrogen can form 3 covalent bonds and 1 coordinate bond. No, nitrogen and phosphorus would not typically form a covalent bond with each.
How many covalent bonds does water have? How many covalent bonds are in ammonia? No, nitrogen and phosphorus would not typically form a covalent bond with each. Water molecules have two simple covalent bonds between one oxygen and two hydrogen. That means it needs 3 bonds. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. Ammonia is a nitrogen atom bonded to three hydrogen atoms. Nitrogen can form 3 covalent bonds and 1 coordinate bond. Nitrogen is in group 5 and therefoe has 5 outer electrons. Would nitrogen and phosphorus form a covalent bond?
Chapter 14 Ionic Bonds 2 41.49/50 = 82.98 3 37.93/50 = 75.86 ppt
That means it needs 3 bonds. How many covalent bonds are in ammonia? Nitrogen can form 3 covalent bonds and 1 coordinate bond. Nitrogen is in group 5 and therefoe has 5 outer electrons. Ammonia is a nitrogen atom bonded to three hydrogen atoms.
PPT Remembering General Chemistry Electronic Structure and Bonding
Water molecules have two simple covalent bonds between one oxygen and two hydrogen. That means it needs 3 bonds. Ammonia is a nitrogen atom bonded to three hydrogen atoms. How many covalent bonds are in ammonia? There is a total of three covalent.
COVALENT BONDING ORBITALS ppt download
How many covalent bonds does water have? Would nitrogen and phosphorus form a covalent bond? Water molecules have two simple covalent bonds between one oxygen and two hydrogen. Nitrogen is in group 5 and therefoe has 5 outer electrons. Nitrogen can form 3 covalent bonds and 1 coordinate bond.
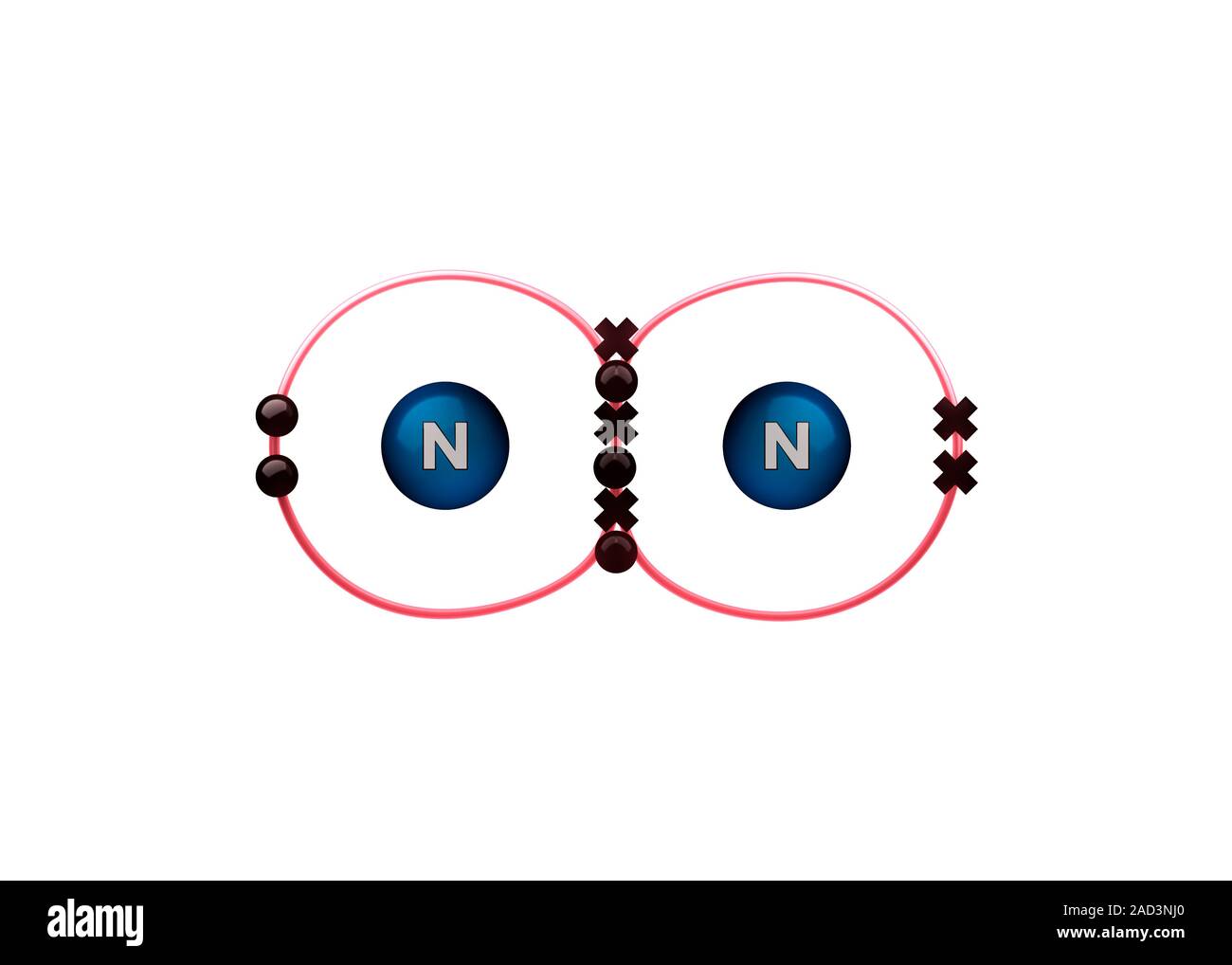
Bond formation in nitrogen molecule. Illustration of the sharing of
Ammonia is a nitrogen atom bonded to three hydrogen atoms. Would nitrogen and phosphorus form a covalent bond? How many covalent bonds does water have? Nitrogen can form 3 covalent bonds and 1 coordinate bond. That means it needs 3 bonds.
Nitrogen Covalent Bond
How many covalent bonds are in ammonia? How many covalent bonds does water have? Ammonia is a nitrogen atom bonded to three hydrogen atoms. No, nitrogen and phosphorus would not typically form a covalent bond with each. Would nitrogen and phosphorus form a covalent bond?
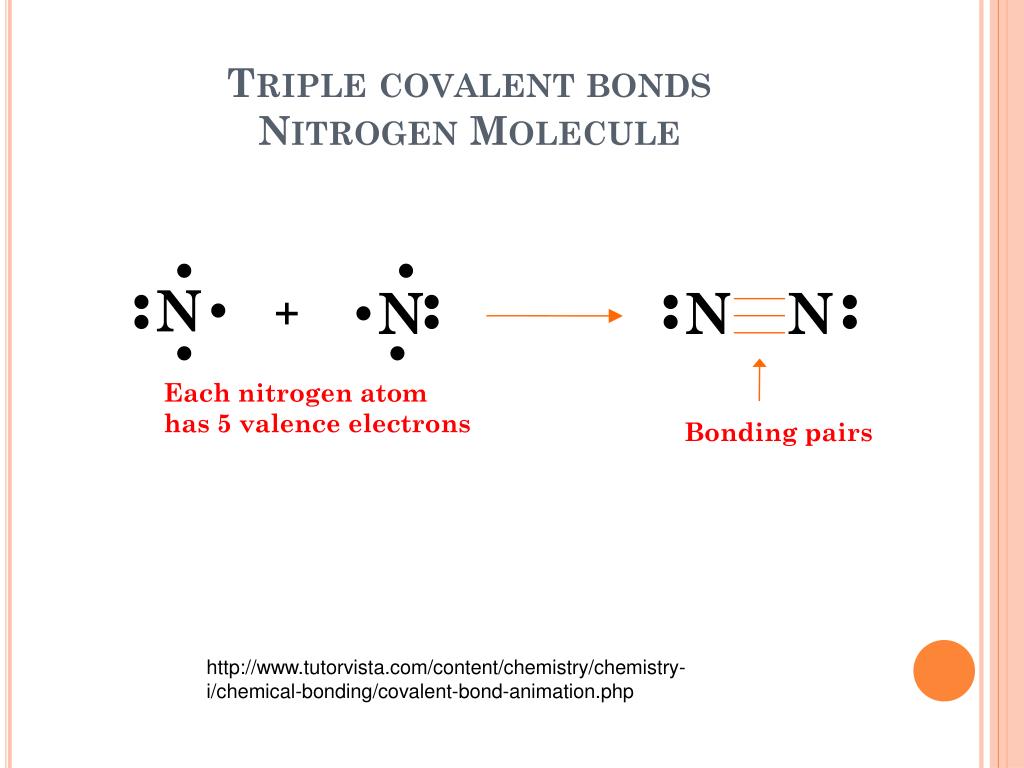
Triple Bond Nitrogen
Nitrogen is in group 5 and therefoe has 5 outer electrons. That means it needs 3 bonds. Would nitrogen and phosphorus form a covalent bond? Water molecules have two simple covalent bonds between one oxygen and two hydrogen. As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons.
The Nitrogen Cycle. ppt download
Would nitrogen and phosphorus form a covalent bond? Ammonia is a nitrogen atom bonded to three hydrogen atoms. How many covalent bonds are in ammonia? How many covalent bonds does water have? Nitrogen can form 3 covalent bonds and 1 coordinate bond.
Noble Gases. ppt download
As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. That means it needs 3 bonds. How many covalent bonds are in ammonia? Nitrogen can form 3 covalent bonds and 1 coordinate bond. There is a total of three covalent.
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236
As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. Nitrogen can form 3 covalent bonds and 1 coordinate bond. Water molecules have two simple covalent bonds between one oxygen and two hydrogen. Nitrogen is in group 5 and therefoe has 5 outer electrons. How many covalent bonds are in ammonia?
PPT Covalent Bonding and Chemical Bonds PowerPoint Presentation, free
As known, nitrogen could form 3 bonds based on octet rule, because it has 5 valence electrons. There is a total of three covalent. No, nitrogen and phosphorus would not typically form a covalent bond with each. How many covalent bonds are in ammonia? Ammonia is a nitrogen atom bonded to three hydrogen atoms.
As Known, Nitrogen Could Form 3 Bonds Based On Octet Rule, Because It Has 5 Valence Electrons.
Would nitrogen and phosphorus form a covalent bond? Ammonia is a nitrogen atom bonded to three hydrogen atoms. That means it needs 3 bonds. No, nitrogen and phosphorus would not typically form a covalent bond with each.
Nitrogen Is In Group 5 And Therefoe Has 5 Outer Electrons.
How many covalent bonds does water have? Nitrogen can form 3 covalent bonds and 1 coordinate bond. There is a total of three covalent. Water molecules have two simple covalent bonds between one oxygen and two hydrogen.