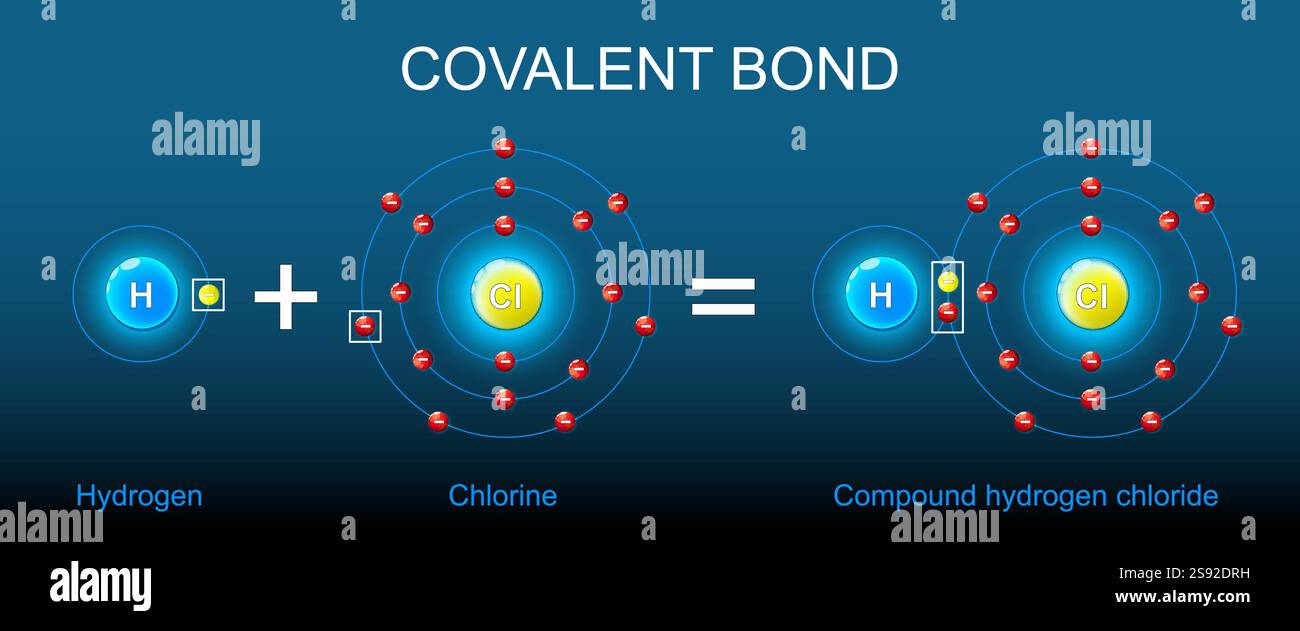
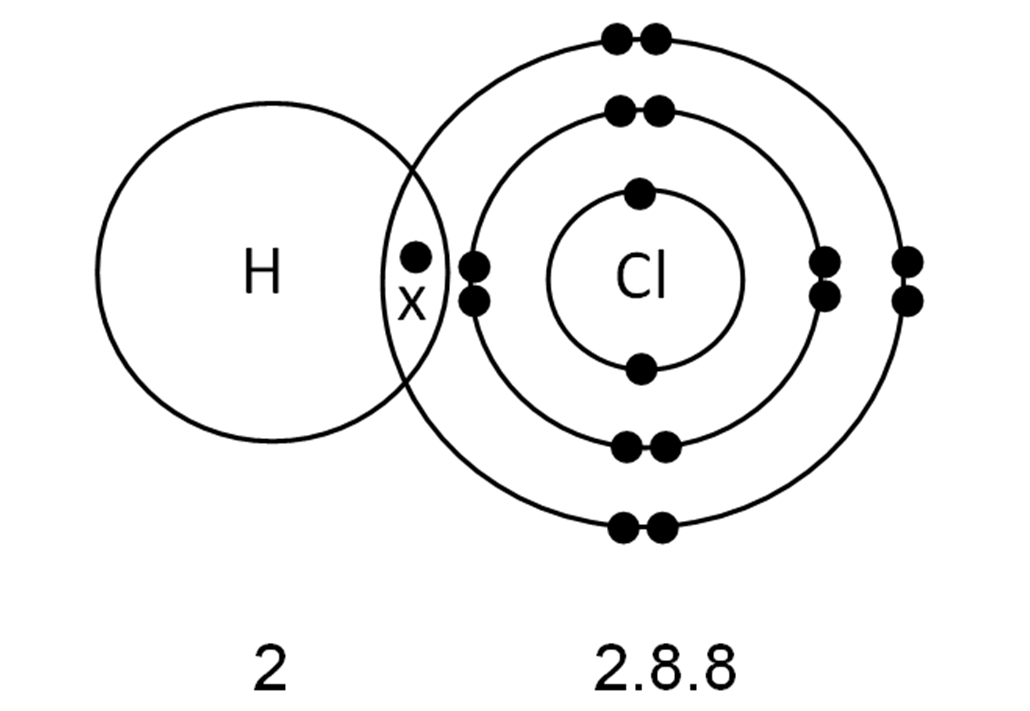
How Is Hydrogen Chloride Formed - In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. The reaction is rapid at. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; Burning of fossil fuels produces.
In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; The reaction is rapid at. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. Burning of fossil fuels produces.
In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; The reaction is rapid at. Burning of fossil fuels produces.
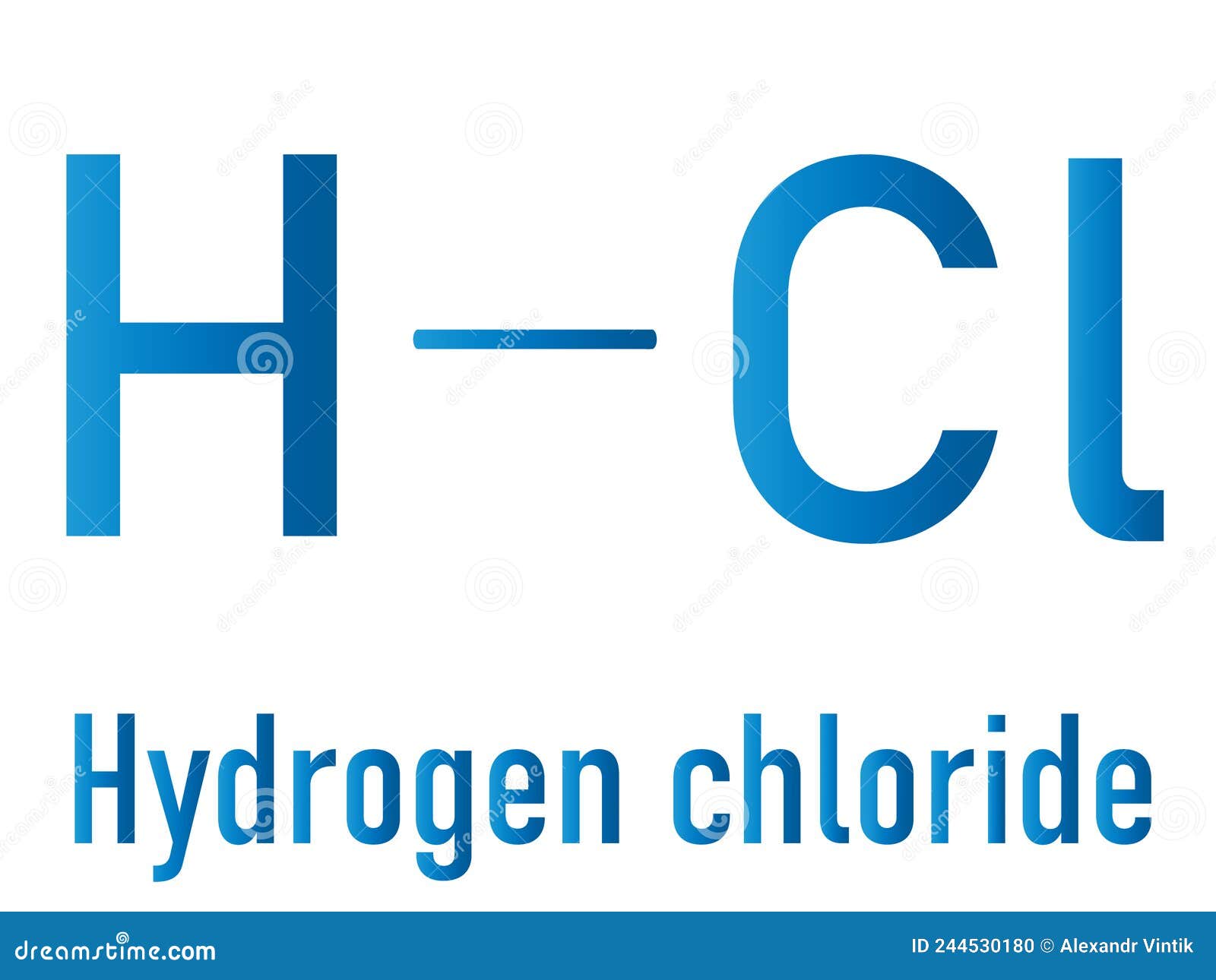
Hydrogen Chloride Lewis Structure
In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. Burning of fossil fuels produces. In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. The reaction is rapid at. Hydrogen chloride may be formed by the.
Hydrogen chloride molecule bond formation Stock Image C028/6479
In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. The reaction is rapid at. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. Burning of fossil fuels produces. Hydrogen chloride may be formed by the.
Chloride Ion Chemical Formula at Darrell Coria blog
Burning of fossil fuels produces. The reaction is rapid at. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. In the chemical reactions, hydrogen atoms on.
Hydrogen Chloride Lewis Structure
Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron,.
Molecular model of hydrogen chloride. Stock Image A504/0048
Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; The reaction is rapid at. In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. Burning of fossil fuels produces. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming.
Science Infographics Vector Illustration Hydrogen Chloride Stock Vector
In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. The reaction is rapid at. Burning of fossil fuels produces. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming.
Pin on Graphics Design Ideas
In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h.
Covalent bond structure. Formation of Compound hydrogen chloride from
Burning of fossil fuels produces. The reaction is rapid at. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming.
Hydrogen Chloride Molecule
In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; Burning of fossil fuels produces. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the.
Hydrogen Chloride Covalent Bond
In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. Burning of fossil fuels produces. In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. Hydrogen chloride may be formed by the direct combination of chlorine (cl.
Burning Of Fossil Fuels Produces.
Hydrogen chloride may be formed by the direct combination of chlorine (cl 2) gas and hydrogen (h 2) gas; In the chemical reactions, hydrogen atoms on the hydrocarbon are replaced by chlorine atoms, whereupon the released hydrogen atom. In hydrogen chloride (hcl), the hydrogen atom loses its only electron, becoming a proton (h⁺), while the chlorine atom gains this electron, forming a. The reaction is rapid at.