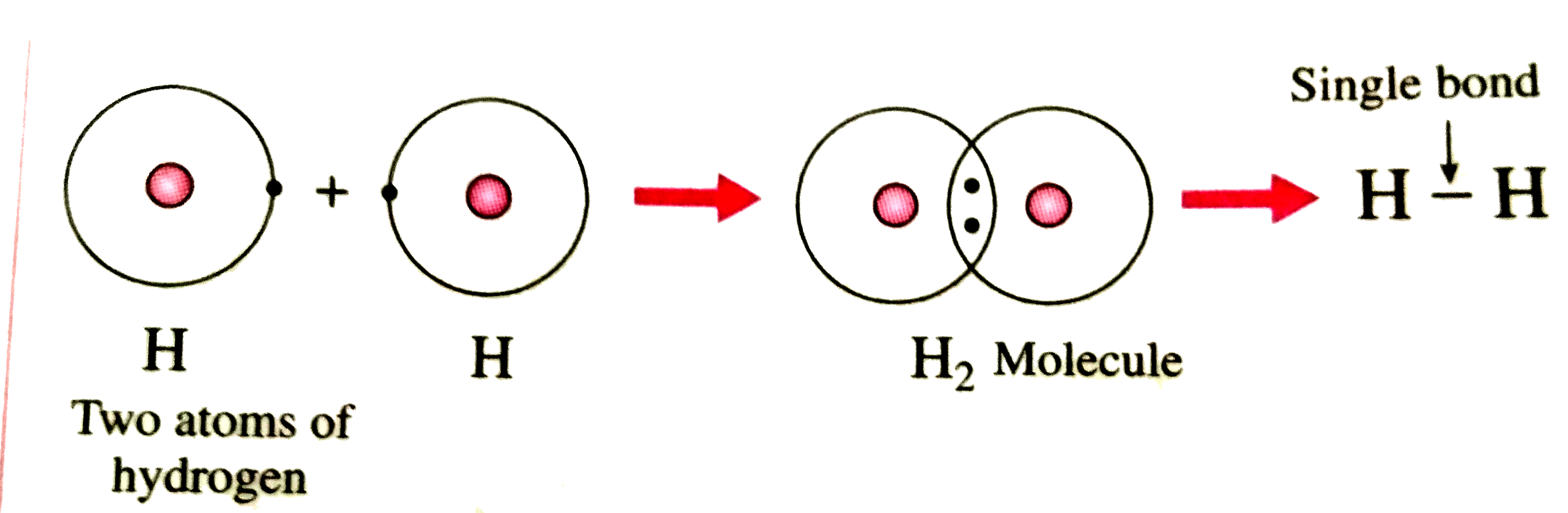
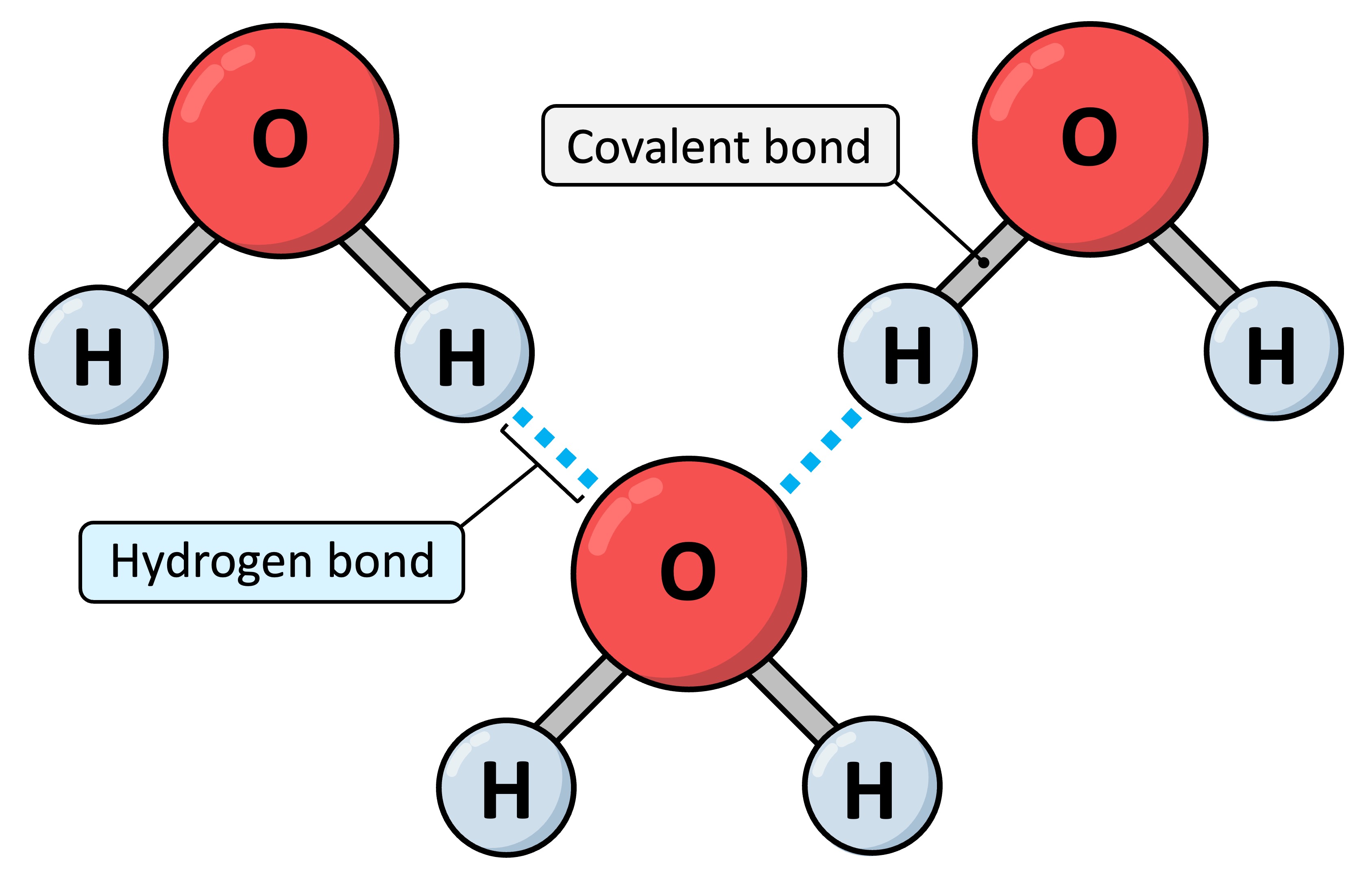
How Is A Hydrogen Bond Formed - The h atom is thus. How does a hydrogen bond form? It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. How is hydrogen bond formed?
Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. The h atom is thus. How is hydrogen bond formed? The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. How does a hydrogen bond form? It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative.
How does a hydrogen bond form? The h atom is thus. How is hydrogen bond formed? Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to.
How is hydrogen molecule formed
It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. The h atom is thus. How does a hydrogen bond form? How is hydrogen bond formed? The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus.
Hydrogen bonds A Simple Explanation of Why They Form
The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. How is hydrogen bond formed? Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. How.
Hydrogen Bonding (ALevel) ChemistryStudent
When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. How is hydrogen bond formed? The h atom is thus. How does a hydrogen bond form? The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus.
Hydrogen Bonding Definition, Types, Effects and Properties (2022)
How is hydrogen bond formed? Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. The h atom is thus. How does a hydrogen bond form?
Biochemistry. ppt download
It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. How does a hydrogen bond form? The h atom is thus. The d atom (usually o, n, f, or.
Hydrogen Bond Definition and Examples
How is hydrogen bond formed? Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. How does a hydrogen bond form? It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. The h atom is thus.
Hydrogen Bonding
How is hydrogen bond formed? The h atom is thus. It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. How does a hydrogen bond form?
Hydrogen bonds A Simple Explanation of Why They Form
When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. How does a hydrogen bond form? The h atom is thus. Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. How is hydrogen bond formed?
Hydrogen Bonds — Overview & Examples Expii
How does a hydrogen bond form? Mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom, forming a bond. The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. The h atom is thus. It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor.
Chapter 2, part A Chemical Principles. ppt download
The h atom is thus. It occurs when a hydrogen (h) atom, covalently bonded to a more electronegative donor atom or group (dn), interacts with another electronegative. How is hydrogen bond formed? The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus. When a hydrogen atom is covalently bonded to an electronegative atom,.
It Occurs When A Hydrogen (H) Atom, Covalently Bonded To A More Electronegative Donor Atom Or Group (Dn), Interacts With Another Electronegative.
How does a hydrogen bond form? How is hydrogen bond formed? When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to. The h atom is thus.
Mainly Through Electrostatic Attraction, The Donor Atom Effectively Shares Its Hydrogen With The Acceptor Atom, Forming A Bond.
The d atom (usually o, n, f, or sometimes s) attracts the electron from the h nucleus.